Environmental signalling in filamentous fungi
Even within the best studied phytopathogenic fungi there is still a great challenge to learn more about how disease is established and propagated. We focus on understanding molecular events of the dynamic processes of environmental signalling in filamentous pathogens during plant-microbe interaction. Receptor or signalling proteins (e.g. histidine kinases), phosphorelay systems or mitogen activated protein kinase (MAPK) cascades play often key roles in signal transduction and even in pathogenicity.
In our research, we use the model organism Magnaporthe oryzae, which is the causal agent of rice blast disease and from the economic and the scientific view one of the most important plant pathogens all over the world. M. oryzae is a suitable organism in our labs because it is hemi-biotrophic, we have detailed knowledge of its infection related morphogenesis and a rich toolbox of methods for directed genetic manipulations.
Fig.1: The infection-related morphogenesis of Magnaporthe oryzae. [A] Attachment of the conidium to the plant surface. [B] Germination of the conidium by sending out a short germ-tube. The apical end of the germ-tube extends and forms a ‘hook’, which is probably involved in sensing chemical and physical features of the surface. [C] Formation of a nascent appressorium. A melanin layer forms in the inner appressorial cell wall during the maturation of the infectious structure. [D] Driven by turgor pressure a penetration peg is forced through the plant cuticle to infect the leaf. [E] Formation of intracellular ‘bulbous’ hyphe. [F] Spread of the infection followed by conidiation.
The high osmolarity glycerol (HOG) signalling pathway in Magnaporthe oryzae
The regulation of cellular turgor in response to environmental changes of osmolarity is one of the most important developments for pathogenic fungi since they have to cope with rapid changing situations within the host. Upon invasion of the plant, the most apparent signal affecting cellular turgor is hyperosmotic stress, which cells content with by generating high concentrations of compatible solutes, e.g. glycerol, to maintain cellular homeostasis. The adjustment of the turgor through the accumulation of these solutes within the cell prevents water loss due to osmosis. Responsible for the detection of and adaption to hyperosmotic stress in fungi is the high osmolarity glycerol (HOG) pathway. We study the molecular basis of osmoregulation in M. oryzae e.g. by means of gene inactivation experiments and characterization of the resulting mutant strains, protein interaction studies, microarray-based transcriptome analysis, RNA-seq analysis (NGS) and proteome analysis.
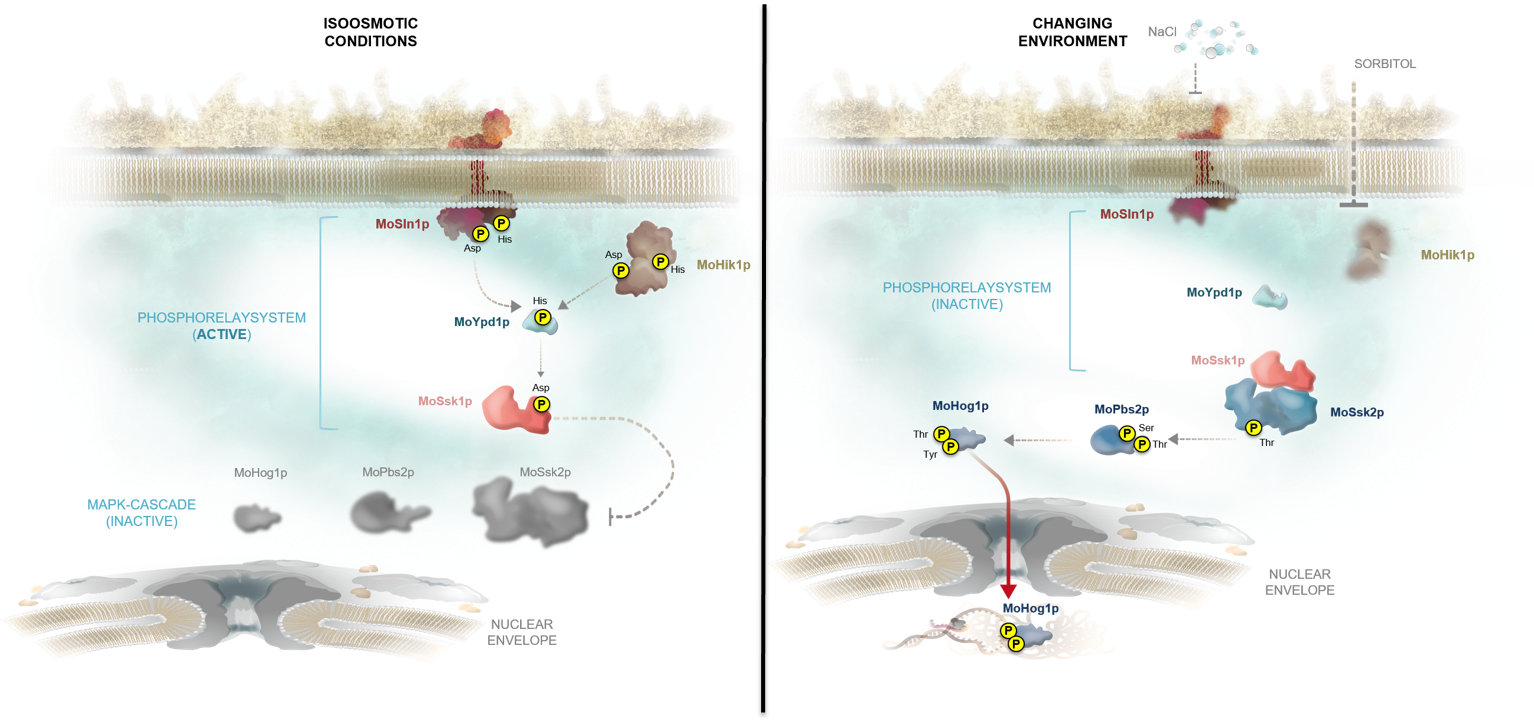
Fig.2: Schematic representation of the Magnaporthe oryzae HOG signaling cascade. The phosphorelay system MoSln1p-MoHik1p-MoYpd1p-MoSsk1p is active (phosphorylated) under normal environmental conditions and the MAPK cascade MoSsk2p-MoPbs2p-MoHog1p is inactive (left). The two-component hybrid histidine kinases (MoSln1p and MoHik1p) are signal sensors for high osmolarity, and the phosphotransferase MoYpd1p mediates the phosphate transfer to MoSsk1p. High osmolarity or salt stress results in dephosphorylation of the signaling components leading to dephosphorylation of MoSsk1p. Therefore, MoSsk1p interacts with MoSsk2, the MAPK cascade MoSsk2p-MoPbs2p-MoHog1p is activated and MoHog1p translocates into the nucleus.
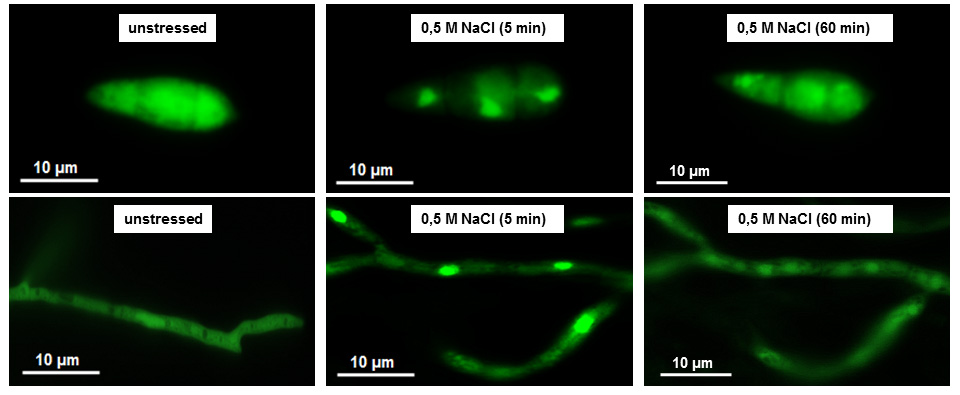 Fig.3: Genetic manipulated Magnaporthe oryzae strain expressing the MoHog1p proteinkinase fused with the green fluorescent protein (GFP). The gfp-signal in the untreated control was found to be homogeneously distributed within the cytosol, whereas this signal strongly accumulates within the three nuclei after osmotic stress (i.e. NaCl, KCl, sorbitol) or fungicide stress (i.e. fludioxonil). Thus, gfp-fused MoHog1p is an excellent biochemical tool analyzing the dynamic processes of osmoregulation in filamentous pathogenic fungi.
Fig.3: Genetic manipulated Magnaporthe oryzae strain expressing the MoHog1p proteinkinase fused with the green fluorescent protein (GFP). The gfp-signal in the untreated control was found to be homogeneously distributed within the cytosol, whereas this signal strongly accumulates within the three nuclei after osmotic stress (i.e. NaCl, KCl, sorbitol) or fungicide stress (i.e. fludioxonil). Thus, gfp-fused MoHog1p is an excellent biochemical tool analyzing the dynamic processes of osmoregulation in filamentous pathogenic fungi.
Analyzing the dynamic processes of environmental signalling by “real-time”-visualization in Magnaporthe oryzae. The video presents the translocation of gfp-fused MoHog1p from the cytosol into the nucleus of M. oryzae upon environmental stress.
Histidine kinases in Magnaporthe oryzae
Whereas the molecular and biochemical basis of the infection-related morphogenesis of M. oryzae on the plant surface has been studied intensively within the last decades, in planta growth and adaption to environmental stresses has not been addressed extensively. Functional histidine kinases are required for plant infection or invasive growth, since their function appears to be essential for the adaption to environmental changes, e.g. osmotic pressure, temperature, nutrition status and reactive oxygen species. Therefore, these signalling molecules also appear to be promising fungicide targets, because they are present in pathogenic organisms whereas absent in mammals. We conducted the first comprehensive functional analysis of all histidine kinases within one phytopathogenic fungus and found interesting candidates for further research in fungal physiology and plant protection.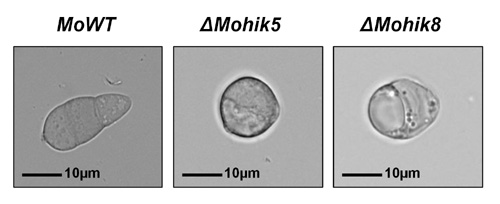
Fig.4: Conidia of the Magnaporthe oryzae strain 70-15 and mutant ΔMohik5 and ΔMohik8. ΔMohik5 and ΔMohik8 are mutants of the M. oryzae strain 70-15, in which the histidine kinase encoding genes MoHIK5 respectively MoHIK8 were inactivated. Conidia of ΔMohik5 and ΔMohik8 were 1-2-celled and almost round whereas the wildtype conidia were typically 3-celled and elongated.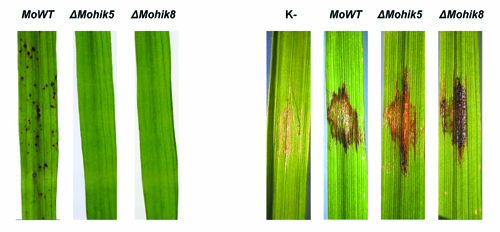
Fig.5: Rice leaves infected with the Magnaporthe oryzae wildtype strain 70-15 and the mutant ΔMohik5 and ΔMohik8. The plant infection assays (left) were carried out with the spray inoculation method. Conidia of M. oryzae and the mutant strains were sprayed on whole rice plants. The studies of in planta growth (right) were conducted due to application of conidia suspensions on wounded rice leaves.
Fig.6: Simplified signal transduction scheme of NaNO2 in Magnaporthe oryzae via the high osmolarity glycerol (HOG) pathway. The hybrid histidine kinases MoSln1p, MoHik1p, MoHik5p and MoHik9p appear to have functions as signal sensors for NaNO2. Nitrite stress (low oxygen levels) causes inhibition of the sensor kinases resulting in phosphorylation and activation of MoHog1p.
Related publications
High osmolarity glycerol (HOG) signaling in Magnaporthe oryzae: Identification of MoYPD1 and its role in osmoregulation, fungicide action and pathogenicity.
S. Jacob,A. Foster, A. Yemelin, E. Thines.
Fungal Biology. 2015, 119 (7), 580–594. doi:10.1016/j.funbio.2015.03.003
Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae.
S. Jacob, A. J. Foster, A. Yemelin, E. Thines.
MicrobiologyOpen. 2014. doi:10.1002/mbo3.197
Visualizing fungicide action: an in vivo tool for rapid validation of fungicides with target location HOG pathway.
S. Bohnert, H. Neumann, E. Thines, and S. Jacob.
Pest Management Science. 2019, 75(3), 772-778. doi:10.1002/ps.5177
